Epidemiology of adolescents living with perinatally acquired HIV infection in a tertiary institution in Lagos State, Nigeria
Introduction
Advancement in HIV care has transformed the disease from an acute potentially fatal to a chronic infection resulting in increased survival of vertically infected adolescents living with HIV infection (1). Adolescents account for 4% of people living with HIV infection worldwide. Overall, 18 million adolescents, aged 10 to 19 years are HIV positive. Of the population of adolescents with HIV infection, 85% live in sub-Saharan Africa. In Nigeria, 240,000 adolescents are living with HIV and sadly, Nigeria is the only country with increase mortality rate in HIV-infected adolescents (2-4).
Adolescents with HIV-infection are a vulnerable group of persons. They are faced with many challenges like poor drug compliance with increase mortality. HIV infection is one of the leading cause of death in adolescents and the global increase in HIV related death was primarily seen in adolescents (3). Achievement of viral suppression of 90% of all people receiving antiretroviral therapy requires a major focus on adolescents living with HIV infection. Non-adherence amongst HIV-infected adolescents is a major issue that must be addressed in order to achieve the targets to end AIDS epidemic by year 2020.
A recent cross-regional cohort analysis of perinatally infected adolescents from many reported that lost to follow-up (LTFU) care was highest amongst subjects in sub-Saharan Africa (5). However, this analysis did not include data from Nigeria- a country with the second highest number of persons with HIV infection and the only country with increase mortality rate in adolescents. This may create lacunae in the knowledge of the current state of HIV infection among adolescents in sub-Saharan Africa, hence the need for this study. We set out to conduct a retrospective study of data of HIV-infected adolescents assessing care at the adolescent HIV clinic of the Lagos State University Teaching Hospital, Ikeja, Lagos since its inception two years ago.
Methods
Study design
This was a retrospective cohort study that reviewed database of perinatally-infected adolescents attending the adolescent HIV clinic of the Lagos State University Teaching Hospital (LASUTH), Ikeja, Lagos State. This study involved data extraction and anonymous sample which required no informed consent.
The authors conducted a retrospective study on data routinely collected on patients with no contact with the subjects at the time of data evaluation. Hence, no consent or ethical approval was obtained for the study. The study was however conducted in accordance with the declaration of Helsinki (6).
Study setting and population
The Adolescent HIV clinic was carved out in January 2017 from the Paediatric HIV clinic which has been in existence since 2007 to meet their peculiar psychosocial and clinical needs in a supportive atmosphere of respect, privacy and understanding. Currently, the clinic runs once a week. It involves the adolescents aged ten to nineteen years.
At enrolment at diagnosis and in adolescent clinic and with subsequent visits, patients’ biodata, general examination findings and laboratory investigations were obtained according to guidelines. Virological diagnosis of HIV was made in subjects that presented below 18 months of age at diagnosis using HIV-DNA PCR of dried blood spots while antibody testing (Determine rapid test) was done in older subjects. Confirmation of reactive antibody testing was done with Western Blot test on another blood sample.
LTFU was defined as failure of patient to attend clinic for at least 180 days and there is no record of death or transfer (7).
Body weight of individual was recorded at enrolment and routinely using a weighing scale. The height was measured with subjects standing barefoot and looking forward using a stadiometer. In subjects enrolled below 2 years, length was recorded using an infantometer.
Height for age (HFA) was assessed at enrolment and on clinic visits. Subjects with HFA Z score <−3 were classified as very stunted and those <−2 to −3 as stunted (8).
Laboratory investigations (CD4 count and viral load) were done at the IHVN Haematology & Virology laboratory dedicated to HIV care. Disease stage was classified into one to four at diagnosis according to World Health Organization criteria (9). Viral load assay is done six months after commencement of HAART and subsequently annually at the hospital’s general haematology laboratory. CD4 count is routinely requested at diagnosis, and subsequently every six months.
Data extraction and management
Baseline sociodemographic, anthropometric, clinical and outcome data were extracted from the Adolescent Clinic Database into a Microsoft Excel 2013 sheet and imported into Statistical Package for Social Sciences (SPSS) version 20 (IBM Inc., Chicago, USA) for statistical analysis.
Descriptive statistics was conducted on the data. Categorical variables were expressed as frequencies and percentages while continuous variables were summarized with mean and standard deviation (if normally distributed) or median with interquartile range (if skewed). Categorical variables were compared with chi square or Fisher exact test. Probability values less than 0.05 at 95% confidence interval was considered statistically significant.
Results
A total number of 273 adolescents were enrolled and transferred to adolescent clinic in LASUTH in the last two years (January 2017 to December 2018). In all, 269 (98.5%) acquired HIV infection through maternal-to-child transmission. A total of 138 (51.3%) of perinatally infected subjects were males with male-to-female ratio of 1.1:1. The subjects had been followed up for a mean duration of 100.6±37.4 months ranging from 7 months to 216 months since diagnosis.
In the total cohort, the median age at diagnosis was 5.0 years (IQR 3.0, 8.0) with a range of two weeks to seventeen years. The median age at diagnosis was similar between boys and girls {[6.00 (IQR 3.00, 8.00) vs. 6.00 (IQR 4.00, 9.00)], Mann-Whitney U-value =−0.255, P=0.799]}.
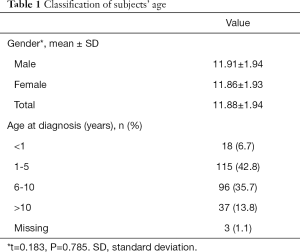
Almost half (49.5%) of the subjects were diagnosed before the sixth birthday.as shown in Table 1.
Full table
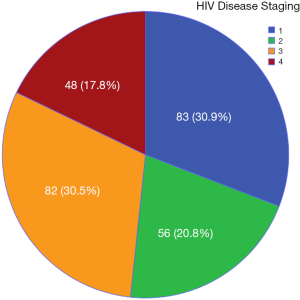
Almost half (48.3%) of the subjects had severe disease (stage 3 or 4) while about one-third were asymptomatic (stage 1) as shown in Figure 1.
The proportion of subjects who were stunted increased with worsening disease (χ2 =43.481, P≤0.0001; 38.228, P=0.0001) as shown in Table 2.

Full table
The median CD4 counts at diagnosis was 497 cells/µL (n=248, IQR 251, 857). Median CD4% was 15%. A significantly higher proportion of subjects with stage 4 disease had CD4% below 15% compared to those with stage 1 disease [27 (61.4%) vs. 18 (22.2%)]. Also, a linear decrease in CD4% was observed with increase disease stage (χ2 =39.532, P=0.001). There was a relationship between CD4 count at diagnosis and disease stage as shown in Table 3.

Full table
The median age of subjects at ARVs commencement was 5.5 years (IQR 2.5, 8.0). The majority [n=229 (85.1%)] and 36 (13.4%) subjects commenced HAART at age below and above ten years, respectively. However, 4 (1.49%) subjects never had HAART
Relationship of ARVs on nutritional status
The median height z-score at enrolment in paediatric HIV clinic was −1.27 (IQR =−2.34, −0.64). At enrolment, 17.7%, 16.5% and 65.8% had severe stunting, stunting and normal height for age respectively. The recent median height z-score of subjects was −1.01 (IQR =−2.06, 2.17). In all, significant increase in proportion of subjects with normal height for age was observed over the two-year period (65.8% vs. 73.1%, χ2 =11.681, P=0.02). There was a linear increase in proportion of subjects with normal height with increase in duration on ARVs as shown in Table 4.

Full table
Forty-one (15.2%) subjects were switched to second line ARVs while 228 (84.8%) were on first line ARVs since enrolment. Out of this 41 subjects, 37 (90.2%) are still in care in our facility.
Out of the 269 enrolled into our adolescent HIV clinic, 218 (81.0%) are still in care. Recent viral load done at least six months after commencement of HAART varied from undetected to 712,717 copies/mL amongst subjects in care (Figure 2). A total of 155 (71.1%) subjects had recent viral load below 200 copies/mL (86 target not detected, 69 copies below 20 to 200 copies/mL). A total of 170 (78.0%) subjects in care are virally suppressed with viral load <1,000 copies/mL. A total of 45 (20.6%) have a recent viral load of ≥1,000 copies/mL and 3 (1.38%) subjects have no recent viral load result.
The recent median CD4 count in 153 subjects that had investigation done was 772 cells per microliter (cells/µL). Minimum and maximum CD 4 count varied between 10 and 9,521 cells/µL.
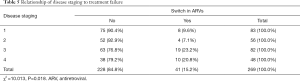
A significantly higher proportion of subjects with stage 3 and 4 disease classification were switched to second line ARVs due to treatment failure compared to those with milder disease as shown in Table 5.
Full table
Overall, 10 (3.71%) mortalities in the subjects enrolled in the study centre. Five of these mortalities occurred in subjects with stage 4 disease. In all, 20 (7.4%) of the subjects were LTFU in care. No relationship was seen between outcome of care and disease stage as shown in Table 6.

Full table
Discussion
Over a two-year period, 269 HIV positive adolescents with vertical transmission of infection were enrolled into our adolescent HIV clinic at Lagos State University Teaching Hospital.
A slightly higher proportion of male adolescents were enrolled compared to female counterparts. This sex distribution is different from reports by Mbalinda et al. (10) in Uganda and a cohort analysis of some sub-Saharan Africa by CIPHER and co-researchers (5) where female predominance was observed.
Equal proportions of subjects were diagnosed between ages five years and below and above five years with a median age at diagnosis of five years. This is slightly higher than 4.1 year with most diagnosed at infancy reported by MaWhinney et al. (11) in Boston. The low percentage of diagnosis in infancy noticed in the present study may reflect lateness of diagnosis of infected children that contributes to high mortality rate.
No significant sex difference for time of diagnosis was noticed in our cohort. In study done in adults, late presentation and diagnosis was reported to be commoner in males (12). Late presentation results in delayed commencement of ARVs, advanced disease and increased mortality.
Almost equal proportion of subjects had stage 1 and 3 disease which were the highest disease staging in the current study. This is in contrast to report by McHugh et al. (13) in Zimbabwe where over-half of affected adolescents had stage 1 and 2 disease. There is need for more effective ways of identifying infected children at an early stage in our environment which will contribute to reduction in morbidity and mortality from HIV infection.
In the present study, subjects diagnosed at older age were more likely to have AIDS than younger subjects. This finding is in keeping with report by Mugavero et al. (14) in North Carolina, USA. Late diagnosis, late commencement of ARVs and advancement of disease increases risk of disease transmission. With advance disease in older age group where risky sexual habits is more likely, the need for early diagnosis as a tool to prevention of transmission of HIV disease cannot be overemphasized. Late diagnosis increases disease progression in individuals with HIV infection (15). It was therefore not surprising in the current study that occurrence of AIDS was commoner in children diagnosed at older ages.
Duration before commencement of ARV drugs after diagnosis ranged between day zero to ten years in the current study. Median age of subjects when ARV was commenced in the current study was 6 years. This late drug commencement is in keeping with report on Cohort analysis of adolescents with perinatal HIV by the collaborative Initiative for Paediatric HIV Education and Research Global Cohort Collaboration and co-authors (5) on some sub-Saharan countries when compared to 0.9 years in North Carolina. Late age at commencement of ARVs is as a result of late diagnosis, a contributor to high mortality in HIV infection. Similar proportion of subjects in the current study commenced ART late when compared with report in the global cohort analysis (5).
Subjects with advanced disease were more likely to be stunted compared to those with stage 1 and 2 diseases. Significant improvement in height was observed following the commencement of ARVs. This finding is not surprising because malnutrition is a common clinical manifestation in HIV infected children and administration of ARVs has been shown to restore growth in affected individuals (16). The finding of malnutrition in about a quarter of subjects at the last visit is worrisome. Similar finding in the global cohort study shows this is not limited to our centre. HIV infection causes growth faltering (17) and its significant importance as a determinant for survival (18) indicates the need for effective and consistent nutritional support of affected individuals.
Majority of adolescents in the present study have been on ARV drugs for less than ten years. A similar finding was documented in all reviewed countries in the global cohort analysis (5). Previous recommendation of WHO on commencement of ARVs at a certain CD4 count as well as late diagnosis will account for the lower period on medication compared to the age at diagnosis (19).
Quite a high proportion of subjects in the current study have been switched to second line ARV therapy due to drug failure and resistance. Over one-fifth of the subjects are not virally suppressed with viral load above 1000 copies/ml. Failure of drug compliance, treatment failure and resistance are major problems in adolescents. This finding in adolescents with high risk of harmful sexual habits, avoidance of disclosure and spread of infection requires urgent attention. In our centre, support group and measures such as reminder on drug administration using text messages and phone calls are measures currently adopted to increase adherence to medications. To achieve treatment target to end AIDS epidemic by the year 2020, effective tools to address treatment failure in adolescents globally cannot be overemphasized.
A relationship between treatment failure and disease staging was established in the current study. This is similar to report by Kyaw and co-researchers (20) in a study done in Myanmar in Asia. Baseline low immunity and high viral load have been shown to reduce response of individuals with HIV to treatment (21).
HIV related mortality in the current study was 3.71%. This is slightly higher than reported value of 2.9% in other sub-Saharan Africa in the cross-regional global cohort study (5). Nigeria is the only country with a rising mortality rate of HIV-related deaths in adolescents (4). There is urgent need for interventions that will effectively reduce these avoidable deaths in this group of individuals.
In conclusion, adolescents with perinatally acquired HIV-infection are on the increase due to increase availability of ARV drugs. This group of individuals are faced with poor drug compliance, treatment failure, high rate of LTFU and death. Improvement in their care is essential in achieving target of ending AIDS in the nearest future. There is also need for a large cohort study of adolescents with HIV infection in Nigeria.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aoi-19-13). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The authors conducted a retrospective study on data routinely collected on patients with no contact with the subjects at the time of data evaluation. Hence, no consent or ethical approval was obtained for the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- UNAIDS. (2014). 90-90-90 An ambitious treatment target to help ends the AIDS epidemic. Available online: . Accessed 9th January, 2019.http://www.unaids.org/sites/default/files/media_asset/90-90-90_en_0
- Avert. Young people, HIV and AIDS. Available online: . Accessed 8th January, 2019.https://www.avert.org
- UNICEF. HIV and AIDS in adolescents. Available online: . Accessed 8th January 2019.https://data.unicef.org
- AVERT. HIV and AIDS in Nigeria. Available online: . Accessed 12th February, 2019.https://www.avert.org
- The Collaborative Initiative for Paediatric HIV Education and Research (CIPHER) Global Cohort Collaboration, Slogrove AL, Schomaker M, et al. The epidemiology of adolescents living with perinatally acquired HIV: A cross-region global cohort analysis. PLoS Med 2018;15:e1002514. [Crossref] [PubMed]
- WMA Declaration of Helsinki—Ethical Principles for Medical Research Involving Human Subjects. Available online: https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/
- Chi BH, Yiannoutsos CT, Westfall AO, et al. Universal definition of loss to follow-up in HIV treatment programs: A statistical analysis of 111 facilities in Africa, Asia and Latin America. PLoS Med 2011;8:e1001111. [Crossref] [PubMed]
- WHO. Physical status: the use and interpretation of anthropometry. Report of a WHO Expert committee. World Health Organ Tech Rep Ser Geneva. 1995.
- WHO. World Health Organization (WHO) case definition of HIV for surveillance and raised clinical staging and immunological classification of HIV-related disease in adults and children. 2006 Geneva, Switzerland. Accessed 3rd January, 2019.
- Mbalinda SN, Kiwanuka N, Kaye DK. Correlates of ever had sex among perinatally HIV-infected adolescents in Uganda. Reprod Health 2015;12:96. [Crossref] [PubMed]
- MaWhinney S, Pagano M, Thomas PA. Age at diagnosis for children with perinatally acquired HIV. J Acquir Immune Defic Syndr 1988;1993:1139-44. [PubMed]
- Jiang H, Yin J, Fan Y, et al. Gender difference in advanced HIV disease and late presentation according to European consensus definition. Sci Rep 2015;5:14543. [Crossref] [PubMed]
- McHugh G, Rylance J, Mujuru H, et al. Chronic morbidity among older children and adolescents at diagnosis of HIV infection. J Acquir Immune Defic Syndr 2016;73:275-81. [Crossref] [PubMed]
- Mugavero MJ, Castellano C, Edelman D, et al. Late diagnosis of HIV infection: the role of age and sex. Am J Med 2007;120:370-3. [Crossref] [PubMed]
- Centers for Disease Control and Prevention (CDC). Late versus early testing of HIV--16 Sites, United States, 2000-2003. MMWR Morb Mortal Wkly Rep 2003;52:581-6. [PubMed]
- Verweel G, van Rossum AM, Hartwig NG, et al. Treatment with highly active antiretroviral therapy in human immunodeficiency virus type 1-infected children is associated with a sustained effect on growth. Pediatrics 2002;109:E25. [Crossref] [PubMed]
- Arpadi SM, Cuff PA, Kotler DP, et al. Growth velocity, fat-free mass and energy intake are adversely related to viral load in HIV-infected children. J Nutr 2000;130:2498-502. [Crossref] [PubMed]
- WHO. Guidelines for an integral approach to the nutritional care of HIV-infected children (6 months-14 years); handbook, chart booklet and guideline for country adaptation. Geneva World Health Organization; 2009. Available online: . Accessed 10th January, 2019.http://www.who.int/nutrition/publications/hivaids/97892
- WHO consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach. Geneva: world Health Organization, 2013. Available online: . Accessed 9th January, 2019.http://www.who.int/hiv/pub/guideline/arv/2013/en
- Kyaw NTT, Harries AD, Kumar AMV, et al. High rate of virological failure and low rate of switching to second-line treatment among adolescents and adults living with HIV on first line ART in Myanmar, 2005-2015. PLoS One 2017;12:e0171780. [Crossref] [PubMed]
- Kamya MR, Mayanja-Kizza H, Kambugu A, et al. Predictors of long-term viral failure among Ugandan children and adults treated with antiretroviral therapy. J Acquir Immune Defic Syndr 2007;46:187-93. [Crossref] [PubMed]
Cite this article as: Adekunle MO, Ubuane PO, Animasahun BA, Afadapa MA, Akinola MA. Epidemiology of adolescents living with perinatally acquired HIV infection in a tertiary institution in Lagos State, Nigeria. Ann Infect 2020;4:1.